
This lesson will examine the two major classes of phototsynthetic pigments, chlorophylls and carotenoids, their biochemical structures and their biosynthesis. The organization of these pigments into photosynthetic pigment, which are protein complexes that harvest light and convert its energy into biochemical energy will be explained.
 |
|
Lesson Navigation Tips:
|
Overview
This lesson will examine the two major classes of phototsynthetic pigments, chlorophylls and carotenoids, their biochemical structures and their biosynthesis. The organization of these pigments into photosynthetic pigments, which are protein complexes that harvest light and convert its energy into biochemical energy will be explained.
Objectives
At the completion of this lesson, students will be able to:
Development of this lesson was supported in part by Cooperative State Research, Education, & Extension Service, U.S. Dept of Agriculture under Agreement Number 98-EATP-1-0403 administered by Cornell University and the American Distance Education Consortium (ADEC). Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. This manuscript has been assigned Journal Series NO. 03-9, College of Agricultural Sciences and natural Resources, University of Nebraska.
A pigment is a generic term for a molecule that absorbs light and has a color. Plants contain many pigments, giving rise to the various colors we see. Flowers and fruits obviously contain a large number of organic molecules that absorb light. Leaves, stems and roots also contain a variety of pigments. Such pigment molecules include anthocyanins, flavanoids, flavines, quinones and cytochromes, just to name a few. However, none of these should be considered a photosynthetic pigment. Photosynthetic pigments are the only pigments that have the ability to absorb energy from sunlight and make it available to the photosynthetic apparatus. In land plants, there are two classes of these photosynthetic pigments, the chlorophylls and the carotenoids.
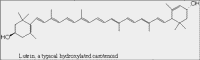 |
| Structure A: Molecular structure of lutein, a carotenoid. |
The ability of chlorophyll and carotenoid molecules to absorb the energy of light and use it effectively is related to their molecular structure and to their organization within the cell. You learned in a previous lesson (The Interaction of Light with Biological Molecules) that pigments absorb the energy from photons through systems of conjugated double bonds. Examine the molecular structure of representative chlorophyll and carotenoid molecules in Figures: Structure A and B.
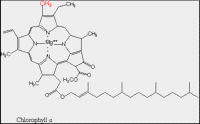 |
| Structure B: Molecular structure of chlorophyll. |
Notice the linear system of conjugated double bonds in the carotenoid (lutein) and the zig-zag of conjugated double bonds in the large ring structure of the chlorophyll. These systems of conjugated double bonds are responsible for the ability to absorb light energy from photons.
Most land plants have two forms of chlorophyll (Chl), designated as Chl a and Chl b. They differ in that Chl a has a methyl (the red -CH3) group on the perimeter of its large ring (called a tetrapyrrole) which is oxidized to form a formyl (-CH=O) group in Chl b. This difference permits these two pigment molecules to absorb slightly different wavelengths of light.
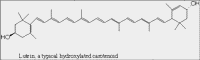 |
| Structure A: Molecular structure of lutein, a carotenoid. |
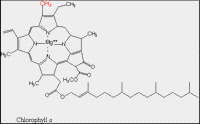 |
| Structure B: Molecular structure of chlorophyll. |
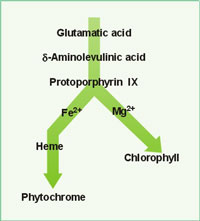 |
| Chlorophyll Pathway: Main components of the chemical pathway which synthesize chlorophyll. |
 |
| Structure A: Molecular structure of lutein, a carotenoid. |
The other class of photosynthetic pigments is the carotenoids. Most land plants contain a variety of carotenoids including beta-carotene, lutein, neoxanthin and violaxanthin. Their basic structure is composed of a repeating, branched five-carbon unit. Molecules formed from these five-carbon units are often called isoprenoids. Notice that the carotenoid structures also contain systems of conjugated double bonds that are responsible for the absorption of light.
 |
| Structure B: Molecular structure of chlorophyll. |
Most carotenoids absorb photons in the blue region of the light spectrum (400 to 500 nm) and appear to be yellow; an exception is the primary red pigment in tomatoes, a carotenoid named lycopene.
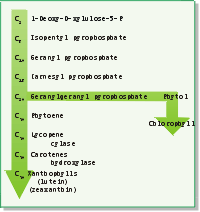 |
| Isoprenoid Pathway: Carotenoid molecules are synthesized in the isoprenoid pathway. |
The synthesis of carotenoids utilizes the isoprenoid pathway (Figure: Isoprenoid Pathway). This pathway is also the basis for the production of such diverse molecules as those involved in aroma molecules, vitamins (e.g. vitamin A), steroids and rubber. The biochemistry involves the addition of successive five-carbon units (or multiples of five carbons) and then rearrangements, cyclizations and additions of functional groups. The pathway is also important in that the geranylgeranyl pyrophosphate intermediate is utilized to make a phytyl group in the biosynthesis of chlorophyll. Carotenoid biosynthesis takes place inside the envelope membranes surrounding the chloroplast, not in the interior membranes containing the chlorophyll.
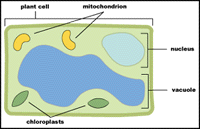 |
| Plant Cell: Location of a few organelles within a plant cell, including the chloroplasts. |
Chlorophyll is a dangerous molecule. This is because it is an excellent photosensitizer and will rapidly cause cell damage when exposed to light. To prevent such damage, the organization and biosynthesis of chlorophyll is carefully controlled. All chlorophyll is located within the chloroplast organelle within the cell.
The chloroplast is surrounded by a pair of membranes called the 'envelope'. The non-membrane, water-soluble material within the chloroplast is called the 'stroma'. Inside the chloroplast, chlorophyll is further confined to a system of membranes called the 'thylakoid membranes'.
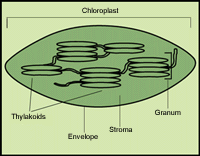 |
| Chloroplast: Chloroplasts are surrounded by envelope membranes and contain a network of membrane structures called thylakoids. These thylakoids can further form structures like stacked coins called grana. Chlorophyll is embedded within these thylakoid membranes. |
Even within the thylakoid membrane system, the chlorophyll molecules do not occur in an unchaperoned state; instead they exist as photosynthetic pigment-protein complexes. The chlorophyll molecules are bound to specific integral membrane proteins along with carotenoids and other components necessary for photosynthesis. The proteins form a structured environment in which the potentially photosensitizing chlorophylls absorb photons, but in which unwanted photodynamic reactions are relatively rare. The pigment-protein complexes are arranged into arrays of hundreds of pigment molecules called photosystems. Because of the spatial and geometric organization, most of the pigments function as light-harvesters and transfer the excitation energy to other pigments before unwanted photochemistry occurs. The carotenoids perform two functions in this environment. First, they harvest light energy and transfer it to chlorophyll molecules. Secondly, they are highly efficient at quenching triplet chlorophylls that are formed before they have a chance to react with molecular oxygen and generate damaging molecules.
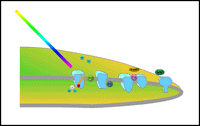 |
| Photosystems: Within the thylakoid membrane are two separate photosystems, Photosystem I (PSI) and Photosystem II (PSII). These function to harness energy from light photons. |
Pigment-protein complexes also contain specific electron transfer components that are important in harnessing energy from the process of photosynthesis. The local organization within the thylakoid membrane is such that there are actually two separate photosystems (See Figure: Photosystems). Each contains an array of antenna chlorophylls and carotenoids.
In the Figure, Antenna, you can see a depiction of the arrangement of antenna pigments within a single photosystem. These pigments represent the majority of the pigment molecules in the photosystems.
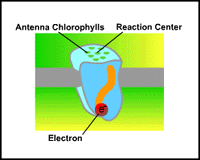 |
| Antenna: Antenna pigments play a vital role in the harnessing of photon energy. |
They function solely to harvest light energy and transfer it to a small number of pigment-protein complexes called reaction centers. In the reaction center, the energy from the photon is used to excite an electron to a higher energy level (also a lower redox potential) so that it can be transferred to an acceptor molecule that has a higher energy level than the original reaction center. The acceptor molecule is then considered to be reduced. In this way, the energy from photons is used to 'pump' electrons to higher energy levels. Each time the reaction center loses an electron, it becomes oxidized and is able to accept electrons from an external source.
Plants evolved to use electrons from water and transfer them to a electron carrier like NADPH for useful biochemistry. The problem is that a single photon does not contain sufficient energy to pump the electron to the required higher energy state in one step. As a consequence, plants use two different photosystems coupled in a series to excite the electrons with two consecutive photons (Figure: Energy Capture). A photosystem is an array of light-harvesting pigments containing a reaction center that carries out the necessary photochemistry. The reaction centers of the photosynthetic photosystems are pigments termed P-680 and P-700 .
 |
| Energy Capture: Plants have developed a schematic with two photosystems. Energy from a photon is first harnessed by Photosystem II. |
Using this greatly simplified scheme, a photon is first captured by the pigments of Photosystem II and the excitation energy transferred to an electron in P-680, a pigment in the reaction center. The high-energy electron in excited P-680 (P-680*) is next transferred to plastoquinone (PQ) via a protein component termed D1. This electron is eventually transferred to Photosystem I. A second photon is similarly captured by Photosystem I and the energy transferred to the pigment P-700 in its reaction center. The electron excited in P-700 is transferred to NADP+ through the protein ferredoxin (Fd) to produce NADPH. When P-680* and P-700* donate their high-energy electrons, they become oxidized (lacking an electron). To bring the system back to neutrality so that it can cycle through the process again, electrons are channeled from water to P-680, thereby forming O2, and electrons from PQ (originally donate by P-680) are transferred to P-700 via cytochrome (Cyt) and plastocyanin (PC) intermediates. The net effect is that the energy in photons is captured in pigments and used to take the electrons from water and pump them to an energy level high enough to form NADPH. The high energy of the electrons in NADPH are used by the cell for many reduction reactions. CLICK HERE to see a simulated animation to better understand this process.
The energy of the electrons in NADPH are not sufficient for anabolic reactions such as CO2 fixation. These processes also require biochemical energy in the form of ATP. The process of photosynthetic electron transfer also generates a gradient of protons (H+) across the photosynthetic membrane (Figure: Proton Gradient).
The transmembrane gradient of protons represents potential chemical energy. The chloroplast thylakoid membrane contains an ATPase complex that is able to convert the trans-membrane proton gradient into the production of ATP (Figure: ATP Formation). This entire process, including the formation of ATP is termed photophosphorylation. CLICK HERE to see this energy transfer in the context of the plant cell. The processes of photosynthetic electron transport and photophosphorylation are used to capture the energy in photons and convert it to high-energy electrons in the form of NADPH and biochemical energy in the form of ATP. With these two molecules, there is sufficient energy to carry out the fixation of CO2 into sugars via the Calvin cycle and to drive the other anabolic reactions of the plant cell. Excess energy formed by photosynthesis is conserved as starch. In the dark, the plant is able to mobilize these sugars and obtain energy by respiration in a fashion similar to the normal processes of microbes and animals.
Light energy in the form of photons is harvested by systems of conjugated double bonds in pigment molecules. Because each pigment can only absorb photons with an energy that exactly matches the amount to excite an electron to an excited state, each pigment has a characteristic color. Both chlorophyll and carotenoid pigments are produced in biosynthetic pathways that also produce other important biomolecules.
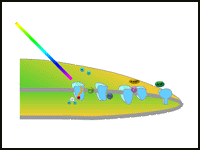 |
| Summary Diagram: Overall illustration of the two photosystems in plants . |
Hans-Walter Heldt 1997 Plant Biochemistry & Molecular Biology. Oxford University Press, Oxford.